Kortison: Béda antarrépisi
Dijieun ku cara narjamahkeun kaca "Cortisone" |
(taya bédana)
|
Révisi nurutkeun 17 Juni 2019 09.02
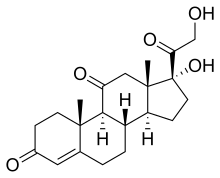
Kortison nyaéta hormon stériod prégnana (21-karbon) anu dikaluarkeun ku kelenjar adrénal dina nyanghareupan setrés. Sacara wangun kimia, ieu sanyawa kortikostéroid anu deukeut jeung kortisol. Kortison biasa dipaké natambaan rupa-rupa nyeri, ku cara neken sistem imun, ku kituna ngurangan peradangan/inflamasi anu dibarung ku nyeri jeung bareuh lebah nu tatu. Lamun dipaké terus-terusan, kortison bisa bahya ogé. [1] [2]
| |
| |
| Ngaran | |
|---|---|
| Pronunciation | /ˈkɔːrtɪsoʊn/, /ˈkɔːrtɪzoʊn/ |
| IUPAC name
(8S,9S,10R,13S,14S,17R)-17-Hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-1,2,6,7,8,9,12,14,15,16-decahydrocyclopenta[a]phenanthrene-3,11-dione
| |
| Other names
17α,21-Dihydroxypregn-4-ene-3,11,20-trione; 17α,21-Dihydroxy-11-ketoprogesterone; 17α-Hydroxy-11-dehydrocorticosterone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.149 |
| KEGG | |
| MeSH | Cortisone |
PubChem <abbr title="<nowiki>Compound ID</nowiki>">CID
|
|
CompTox Dashboard (<abbr title="<nowiki>U.S. Environmental Protection Agency</nowiki>">EPA)
|
|
| |
| |
| Pasipatan | |
| C21H28O5 | |
| Molar mass | 360.450 g·mol−1 |
| Melting point | 220 to 224 °C (428 to 435 °F; 493 to 497 K) |
| Parmakologi | |
| H02AB10 (WHO) S01BA03 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pangaruh jeung mangpaat
Kortison, glukokortikoid, jeung épinéfrin (adrénalin) ngaronjatkeun tekanan getih sarta nyiapkeun awa pikeun réspon lawan atawa ngejat.
Injéksi kortison bisa dipaké pikeun natambaan nyeri & bareuh saharitaeun dina peradangan sendi, urat, atawa bursa, upamana dina tuur, siku, jeung taktak.[1]
Kortison ogé bisa dipaké pikeun ngurangan réspon imun jalma anu boga kasakit otoimun atawa nalika natambaan cangkok organ pikeun nyegah panolakan cangkokan. Suprési sistem imun ogé penting dina ngubaran kondisi peradangan. [3]
Rujukan
- ↑ a b "Cortisone shots". MayoClinic.com. Diakses tanggal July 31, 2013.
- ↑ "Prednisone and other corticosteroids: Balance the risks and benefits". MayoClinic.com. Diakses tanggal 2017-12-21.
- ↑ Driver, Catherine; Shiel, William. "Cortisone Injection (Corticosteroid Injection) of Soft Tissues & Joints". MedicineNet.com. Diakses tanggal August 7, 2013.
Bacaeun salajengna
- Bonagura J., DVM et al. (2000). Current Veterinary Therapy 13. pp. 321–381.
- Ingle DJ (October 1950). "The biologic properties of cortisone: a review". J. Clin. Endocrinol. Metab. 10 (10): 1312–54. doi:10.1210/jcem-10-10-1312. PMID 14794756. http://jcem.endojournals.org/cgi/pmidlookup?view=long&pmid=14794756.
- Woodward R. B.; Sondheimer F.; Taub D. (1951). "The Total Synthesis of Cortisone". Journal of the American Chemical Society 73 (8): 4057. doi:10.1021/ja01152a551.