Arginin
Arginin nyaéta asam amino anu rumusna (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. Molekulna boga gugus guanidino anu napel dina wangun baku asam amino. Dina pH pisiologis, asam karboksilat ngalaman deprotonasi (−CO2−), anapon gugus amino jeung guanidino diprotonasi, ngahasilkeun hiji kation. Ngan énantiomér l-arginin (lambang Arg atawa R) anu kapanggih alami.[1] Résidu Arg mangrupakeun komponén galib dina protéin, disandi ku kodon CGU, CGC, CGA, CGG, AGA, jeung AGG.[2] Gugus guanidin dina arginin téh prékursor pikeun biosintésis oksida nitrat.[3] Sakumaha galibna asam amino, padetanana bodas jeung leyur dina cai.
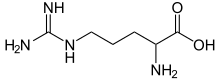 Rumus rangka arginin
| |||
| |||
| Wasta | |||
|---|---|---|---|
| Wasta lian
Asam 2-amino-5-guanidinopentanoat
| |||
| Pananda | |||
Modél 3D (JSmol)
|
|||
| 1725411, 1725412 D, 1725413 L | |||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider | |||
| DrugBank |
| ||
| ECHA InfoCard | 100.000.738 | ||
| Nomer EC | 230-571-3 | ||
| 364938 D | |||
| |||
| KEGG |
| ||
| MeSH | Arginine | ||
PubChem CID
|
|||
| UNII |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Sipat | |||
| C6H14N4O2 | |||
| Massa molar | 174204 g·mol−1 | ||
| Panampilan | Kristal bodas | ||
| Bau | Odourless | ||
| Titik lebur | 260 °C; 500 °F; 533 K | ||
| Titik golak | 368 °C (694 °F; 641 K) | ||
| 14.87 g/100 mL (20 °C) | |||
| Solubilitas | rada leyur dina ethanol teu leyur dina étil éter | ||
| log P | −1.652 | ||
| Kaasaman (pKa) | 2.18 (karboksil), 9.09 (amino), 13.8 (guanidino) | ||
| Thermochemistry | |||
Kapasitas panas (C)
|
232.8 J K−1 mol−1 (at 23.7 °C) | ||
Éntropi
molar baku (S |
250.6 J K−1 mol−1 | ||
Éntalpi baku
formasi (ΔfH⦵298) |
−624.9–−622.3 kJ mol−1 | ||
Éntalpi baku
pembakaran (ΔcH⦵298) |
−3.7396–−3.7370 MJ mol−1 | ||
| Farmakologi | |||
| B05 S | |||
| Baya | |||
| Lambar data kasalametan (SDS) | |||
| Piktogram GHS | 
| ||
| Kecap sinyal GHS | WARNING | ||
| H319 | |||
| P305+P351+P338 | |||
| Dosis atawa konséntrasi létal (LD, LC): | |||
LD50 (dosis médian)
|
5110 mg/kg (beurit, oral) | ||
| Sanyawa patali | |||
Related asam alkanoat
|
|||
Sanyawa patali
|
|||
Iwal disebutkeun séjén, data nu dipidangkeun keur matéri dina kaayaan baku (dina 25 °C, 100 kPa). | |||
| Rujukan kotak info | |||
Baca ogé
édit- Arginin glutamat
- AAKG
- Kanapanin jeung kanalin, analog toksik arginin jeung ornitin.
Rujukan
édit- ↑ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Diarsipkan dari versi asli tanggal 9 October 2008. Diakses tanggal 5 March 2018. Archived 9 Oktober 2008 di Wayback Machine
- ↑ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Diarsipkan dari versi asli tanggal 29 May 2007. Diakses tanggal 2007-05-17.
- ↑ Ignarro LJ (2000-09-13). Nitric Oxide: Biology and Pathobiology. Academic Press. p. 189. ISBN 978-0-08-052503-7.
- Griffiths JR, Unwin RD (2016). Analysis of Protein Post-Translational Modifications by Mass Spectrometry. John Wiley & Sons. ISBN 978-1-119-25088-3.
Tutumbu kaluar
édit| Wikimedia Commons mibanda média séjénna nu patali jeung Arginine . |
| Artikel ieu mangrupa taratas, perlu disampurnakeun. Upami sadérék uninga langkung paos perkawis ieu, dihaturan kanggo ngalengkepan. |