Asam sulfat: Béda antarrépisi
Dijieun ku cara narjamahkeun kaca "Sulfuric acid" |
(taya bédana)
|
Révisi nurutkeun 3 Nopémber 2019 05.26
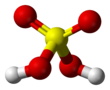
Asam sulfur( alternatif ejaan asam sulfat ), ogé katelah vitriol, nyaéta asam mineral anu diwangun ku unsur walirang, oksigén sareng hidrogén, kalayan rumus molekular H 2 SO <sub id="mwLA">4</sub> . Asam sulfur nyaéta mangrupakeun cairan anu teu aya warnaan (bening), bau, sareng bentukna siga sirop anu leyur dina cai sareng disintésis dina réaksi anu kacida éksotérmik. [5]
| |||

| |||

| |||
| Wasta | |||
|---|---|---|---|
| Wasta IUPAC
Sulfuric acid
| |||
| Wasta lian
Oil of vitriol
| |||
| Pananda | |||
Modél 3D (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.763 | ||
| Nomer EC | 231-639-5 | ||
| Nomer E | E513 (pangatur kaasaman, ...) | ||
| 2122 | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| Nomer UN | 1830 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Sipat | |||
| H2SO4 | |||
| Massa molar | 98.079 g/mol | ||
| Panampilan | Clear, colorless liquid | ||
| Bau | odorless | ||
| Dénsitas | 1.8302 g/cm3, liquid[1] | ||
| Titik lebur | 10.31[1] °C (50.56 °F; 283.46 K) | ||
| Titik golak | 337[1] °C (639 °F; 610 K) When sulfuric acid is above 300 °C (572 °F), it gradually decomposes to SO3 + H2O | ||
| miscible, exothermic | |||
| Tekanan uap | 0.001 mmHg (20 °C)[2] | ||
| Kaasaman (pKa) | −3, 1.99 | ||
| Basa konjugat | Hydrogen sulfate | ||
| Viscosity | 26.7 cP (20 °C) | ||
| Thermochemistry | |||
Éntropi
molar baku (S |
157 J·mol−1·K−1[3] | ||
Éntalpi baku
formasi (ΔfH⦵298) |
−814 kJ·mol−1[3] | ||
| Baya | |||
| Lambar data kasalametan (SDS) | |||
| Piktogram GHS | 
| ||
| Kecap sinyal GHS | Danger | ||
| H314 | |||
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |||
| NFPA 704 | |||
| Titik hurung | Non-flammable | ||
| Dosis atawa konséntrasi létal (LD, LC): | |||
LD50 (dosis médian)
|
2140 mg/kg (rat, oral)[4] | ||
LC50 (konséntrasi médian)
|
50 mg/m3 (guinea pig, 8 hr) 510 mg/m3 (rat, 2 hr) 320 mg/m3 (mouse, 2 hr) 18 mg/m3 (guinea pig)[4] | ||
LCLo (panghandapna dipublikasi)
|
87 mg/m3 (guinea pig, 2.75 hr)[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1 mg/m3[2] | ||
REL (Recommended)
|
TWA 1 mg/m3[2] | ||
IDLH (Immediate danger)
|
15 mg/m3[2] | ||
| Sanyawa patali | |||
Related strong acids
|
Selenic acid Hydrochloric acid Nitric acid Chromic acid | ||
Sanyawa patali
|
Sulfurous acid Peroxymonosulfuric acid Sulfur trioxide Oleum | ||
Iwal disebutkeun séjén, data nu dipidangkeun keur matéri dina kaayaan baku (dina 25 °C, 100 kPa). | |||
| Rujukan kotak info | |||
Karat na bisa digambarkeun ka na asam kuat alami, sarta di konsentrasi anu ageung, digambarkeun ka na sipat penguapan sareng oksidasi na. Asam sulfur oge gaduh sipathygroscopic, nyaeta gampang nyerep uap cai ti hawa . [5] Nalika aya hubungan, asam walirang tiasa nyababkeun kaduruk kana kulit; bahaya pisan bahkan dina konsentrasi sedeng. [6] [7] Alat pikeun ngukur konsentrasi asam sulfur biasana disebut pH meter, nyaéta alat pikeun ngukur darajat kaasaman suatu larutan, di mana pH diturunkeun dina konsentrasi ion Hidrogén.
- ↑ a b c Haynes, William M. (2014). CRC Handbook of Chemistry and Physics (95 ed.). CRC Press. pp. 4–92. ISBN 9781482208689. Diakses tanggal 18 November 2018.
- ↑ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0577". National Institute for Occupational Safety and Health (NIOSH).
- ↑ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 978-0-618-94690-7.
- ↑ a b c Citakan:IDLH
- ↑ a b "Sulfuric acid safety data sheet" (PDF). arkema-inc.com. Diarsipkan dari versi asli (PDF) tanggal 17 June 2012.
Clear to turbid oily odorless liquid, colorless to slightly yellow.
- ↑ "Sulfuric acid – uses". dynamicscience.com.au. Diarsipkan dari versi asli tanggal 9 May 2013.
- ↑ "BASF Chemical Emergency Medical Guidelines – Sulfuric acid (H2SO4)" (PDF). BASF Chemical Company. Diakses tanggal December 18, 2014.